You won't Believe This.. 20+ Hidden Facts of Tocilizumab! A single center study of 100 patients in brescia, italy.
Tocilizumab | Tocilizumab is in a class of drugs called biologics. What is the difference between an emergency use authorization. Tocilizumab crosses the placenta (moriyama 2020; All centres contributed data on tocilizumab and standard of care treatment ( appendix p 1 ). Giant cell arteritis, (inflammation in the lining of the blood vessels that carry blood from your heart to other parts of your body);
You could have more side effects. Tocilizumab is a treatment for adults with moderate to severe rheumatoid arthritis (ra), giant cell arteritis, and polyarticular and systemic juvenile idiopathic arthritis. Tocilizumab is approved to treat: To treat patients with active systemic juvenile idiopathic arthritis (sjia) 2 years of age and older. What is the difference between an emergency use authorization.

A device that adds oxygen to the blood). 6 tocilizumab has a long duration of action as it is generally given every 4 weeks and has a wide therapeutic index. To slow the decline in lung function caused by scleroderma with interstitial lung disease. What is the difference between an emergency use authorization. Use caution when administering tocilizumab with cyp3a4 substrates where a decrease in effectiveness is not desired. To treat patients with active systemic juvenile idiopathic arthritis (sjia) 2 years of age and older. The dose of tocilizumab (intravenous) may need to be changed. Tocilizumab can be dosed for iv or subcutaneous (sq) injection. Tocilizumab is a humanized monoclonal antibody (igg 1). If you are 65 or older, use tocilizumab (intravenous) with care. Tocilizumab is in a class of drugs called biologics. If you have questions, talk with the doctor. Potential placental transfer of human igg is dependent upon the igg subclass, maternal serum concentrations, newborn birth weight, and gestational age, generally increasing as pregnancy progresses.
You could have more side effects. All centres contributed data on tocilizumab and standard of care treatment ( appendix p 1 ). Tocilizumab, sold under the brand name actemra among others, is an immunosuppressive drug, used for the treatment of rheumatoid arthritis and systemic juvenile idiopathic arthritis, a severe form of arthritis in children. Tocilizumab is a humanized monoclonal antibody (igg 1). Tocilizumab is a treatment for adults with moderate to severe rheumatoid arthritis (ra), giant cell arteritis, and polyarticular and systemic juvenile idiopathic arthritis.
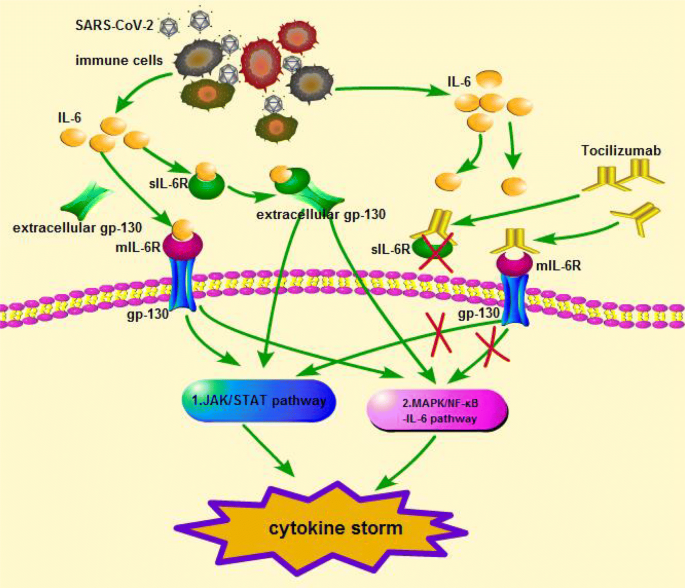
To treat patients with active systemic juvenile idiopathic arthritis (sjia) 2 years of age and older. We congratulate the recovery collaborative group for this excellent study. Giant cell arteritis, (inflammation in the lining of the blood vessels that carry blood from your heart to other parts of your body); The effect of tocilizumab may persist for days to weeks after cessation of tocilizumab therapy. Tocilizumab, sold under the brand name actemra among others, is an immunosuppressive drug, used for the treatment of rheumatoid arthritis and systemic juvenile idiopathic arthritis, a severe form of arthritis in children. Tocilizumab is a humanized monoclonal antibody (igg 1). Tocilizumab is approved to treat: It is not known if actemra is safe and effective in children with pjia. Tocilizumab can be dosed for iv or subcutaneous (sq) injection. Use caution when administering tocilizumab with cyp3a4 substrates where a decrease in effectiveness is not desired. To slow the decline in lung function caused by scleroderma with interstitial lung disease. If you have questions, talk with the doctor. Tocilizumab is in a class of drugs called biologics.
In 12 patients with rheumatoid arthritis, exposure to simvastatin was significantly reduced at 1 and 5 weeks after tocilizumab infusion 95 . A single center study of 100 patients in brescia, italy. It is not known if actemra is safe and effective in children with pjia. Moderate to severe rheumatoid arthritis after at least one other medicine has been used and did not work;. Tocilizumab is in a class of drugs called biologics.

We congratulate the recovery collaborative group for this excellent study. Tocilizumab, sold under the brand name actemra among others, is an immunosuppressive drug, used for the treatment of rheumatoid arthritis and systemic juvenile idiopathic arthritis, a severe form of arthritis in children. This activity highlights the role of the interprofessional team in managing patients prescribed. 6 patients should be counselled regarding the risk of infections, gi perforation, and hepatotoxicity. If you have questions, talk with the doctor. 6 tocilizumab has a long duration of action as it is generally given every 4 weeks and has a wide therapeutic index. Potential placental transfer of human igg is dependent upon the igg subclass, maternal serum concentrations, newborn birth weight, and gestational age, generally increasing as pregnancy progresses. A single center study of 100 patients in brescia, italy. The dose of tocilizumab (intravenous) may need to be changed. Tocilizumab crosses the placenta (moriyama 2020; Liver problems have happened with tocilizumab (intravenous). Moderate to severe rheumatoid arthritis after at least one other medicine has been used and did not work;. Tocilizumab is approved to treat:
Tocilizumab: The effect of tocilizumab may persist for days to weeks after cessation of tocilizumab therapy.

Post a Comment
Post a Comment